5/11/2009
2 octopus babies in tank. Appear to be in different developmental stages (one is very red, lots of chromatophores, the other is almost pure white). Possibly hatched because agitated by mom.
4/30/2009 -
Developed Western started yesterday according to Western Breeze protocol.
4/29/2009
Protein gel for Western:
Protein was extracted from all leg tissue using 500uL CellLytic
Samples loaded into wells: 20 uL (protein+water) and 20 uL 2X SDS. All sample volumes calculated to contain 9.7 ug of protein based on Bradford absorbance data.
| Sample |
Absorbance |
ug/mL |
ug/uL |
| Leg 3/4 |
0.1015 |
980 |
0.98 |
| Leg 4/1 |
0.124666667 |
1211.666667 |
1.211666667 |
| Leg 10/24 |
0.046667 |
485.1888889 |
0.485188889 |
| Leg 2/18 |
0.104 |
1122.222222 |
1.122222222 |
| Leg 2/4 |
0.033333 |
337.0333333 |
2.8 |
| Leg 2/25 |
0.095 |
1022.22 |
1.022 |
| Leg 2/11 |
.009 |
66.67 |
0.4 |
Protein content based on lowest concentration sample: 10/24=.485 ug/ul
.485 ug/ul x 20 ul= 9.7 ug protein
Volumes of remaining samples:
9.7 ug protein / 1.211 ug/ul = 8 uL of leg protein sample
Water to 20 uL (I.e 20-8 ul of sample= 16 ul water)
Loaded into wells: 20 ul of sample+water and 20 ul 2X SDS
Mucus samples are 20 uL liquid (composed of seawater and mucus) and 20 uL 2X SDS.
1-ladder
2- 20 uL leg protein (9.7 ug) 10/24
3- 3.5 uL leg protein (9.7 ug) 2/4 + 16.5 uL water
4- 20 uL leg protein (9.7 ug) 2/11
5- 8.7 uL leg protein (9.7 ug) 2/18 + 11.3 uL water
6- 9.7 uL leg protein (9.7 ug) 2/25 + 10.3 uL water
7- 9.7 uL leg protein (9.7 ug) 3/4 + 10.3 uL water
8- 8.1 uL leg protein (9.7 ug) 4/1 + 11.9 uL water
9- mucus 1/28
10- mucus 2/11
11- mucus 3/4
12- mucus 4/8
45 min, 150 volts (incubates at 100 degrees, then centrifuged 2 min at max speed)
Gel equilibrated in transfer buffer for 15 minutes, transfer completed at 20 V for 30 min.
Sam took over from here
-
Using Invitrogen Western Breeze kit (anti-mouse). Blocked membrane for 30mins according to protocol. Added 3uL (1:3000 dilution) of anti-HSP70 Ab and incubate membrane O/N @ 4C with shaking.
The gel was Coomassie stained for 5mins and then destained O/N w/ 10% acetic acid with shaking.
4/27/2009
Started prep for Western (will run gel on Wed.) Concentrated two leg protein samples with lowest protein content (2/4 and 2/11) using centricon filter device.
4/24/2009
Extracted protein from final two leg samples (3/4, 4/1), Bradford.
4/22/2009
Repeat of PCR from 4/20, did not work. Possible problem with Syto 13.
4/20/2009
PCR using cDNA from 4/17
4/17/2009
DNAse treatment, cDNA
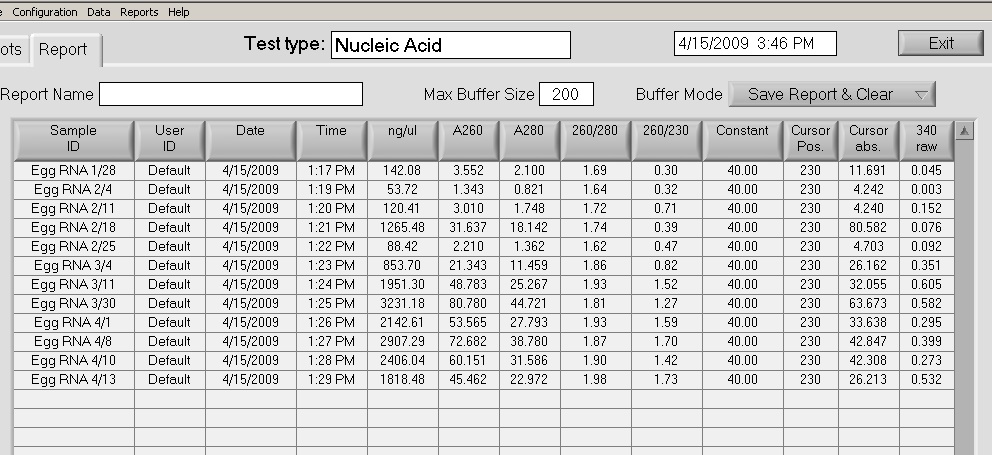
4/15/2009
Completed RNA isolation
4/13/2009
Began RNA isolation on 12 egg samples, used approximately 10 eggs/tube
4/10/09
Attempted to bleed octopus using different method, didn't work (octopus too small to use method described in paper). Collected eggs and took pictures under scope. Finished entering data from webcam into spreadsheet.
Will continue sampling eggs, but not mucus or leg (for now). Eggs collected every Mon/Wed/Fri
4/8/09
Attempted to bleed octopus, did not work.
Collected mucus and eggs (in -80), looked at eggs under scope, small octopus are clearly visible inside. Will get picture.
4/6/09
Fed octopus
Eggs look the same, will take sample today
Observation: rough parts on skin (papillae? were not there before.)
http://genefish.fish.washington.edu/~steven/Octopus%20timelapse/
4/1/09
To Do:
Continue sampling?
-Eggs (3 times/week)
-Leg samples?
-Get weight
Behavior analysis (go through pictures from web cam, check boxes...)
Egg development (gene expression)
Octopus senescence (proteins, gene expression in legs?)
Today: collected sample of eggs, leg, mucus from skin and mucus from bucket.
Behavior same as previous sampling, fought off net attempting to stay on top of eggs. Still eating (ate crab from Mon. 3/30), color looks more dull, but still red. Attempted to weigh, but scale read 108 g, which is about 10 g more than first weight that was taken (I don't think any of these weights have been accurate, scale is not sensitive enough, or octopus moving around in bucket disrupts reading).
3/30/09
Fed octopus crab, noticed dark spots on eggs. Collected small strand of eggs to look at more closely. Appears to be 2 red spots on each egg near the base (point of attachment). See picture.
3/12/09
Ran PCR with primers for eggs, results inconclusive (picture?)
3/11/09
Designed primers, ordered (arrive Thurs. AM)
Started poster for NSA (finished by Mon.)
Sampled: Didn't get an accurate weight
No leg
2 mucus samples and eggs
3/10/09
DNAase treated egg RNA (5 total samples), made cDNA:
6 uL RNA sample, 14 uL master mix
3/6/09
Completed RNA isolation on octopus eggs, re-suspended in 10uL water (in -80, Shellfish box 5)

3/4/2009
Isolate RNA from egg samples: 5 total samples, used 3 eggs per tube
Sampled: Eggs, leg, 2 mucus
3/2/2009
Standard curve in excel, protein concentrations of samples
Bradford Results
2/27/2009
Bradford: on all samples (17 total), mucus from suction/mucus from bucket/extracted protein from legs
2/25/2009
Sampling today: Leg, eggs, mucus from bucket, mucus from skin, attempted to collect mucus from skin once octopus back in tank (partially underwater). Weight: approximately 91 g (I think this is more accurate than weight from 2/18).
Extracted protein from legs: 10/24-2/25. 500 uL cell lytic, ground up (not completely dissolved), centrifuge 10 min. at max speed.
Bradford on Friday (all mucus samples, extracted protein from legs)
2/23/2009
Concentrated 6 total mucus samples using centricon (YM-3). Only mucus samples taken from bucket (lots of seawater). 4000 RPM for 30 min.
Mucus was collected from bottom of sampling bucket after octopus removed: Sampling dates of mucus concentrated...
11/21, 1/21, 1/28, 2/4, 2/11, 2/18
Approximately 100 uL collected from each filtration. Originally, each tube has about 1 mL of liquid (seawater and mucus). Put in new tubes, labeled w/ date.
Fed octopus: She has piled gravel and mussel shells underneath the corner with the eggs, now moon snail shell is on top of pile. Has been in same position every time I have fed her for the last week or so. Her head is pressed up against the eggs, with underside of body (with mouth) facing out towards the tank. When I put crab in tank, she ignores it. But it appears that she eats them eventually because there are no live crabs in the tank (Will take picture).
2/18/2009
Sampled:
Leg
Mucus from bucket
Mucus from skin
Eggs
Weight=approximately 88 g (kept moving around in bucket, scale fluctuated)
Much easier to catch. Was in corner with eggs, but when I tried to grab her with the net, she swam away into the water. Was easy to scoop her up from there. Once in bucket, tried to escape constantly (seems more active today then last week).
2/13/2009
Began RNA extraction on 3 leg samples (tubes in -20, labeled 1,2,3)
1: Leg sample taken on 10/24
2: Leg sample taken on 2/4
3: Leg sample taken on 2/11
Added isopropanol, then stopped.
*Should not be extracting RNA from legs, was suppose to do eggs*
Won't continue extraction process, but tubes are in freezer.
2/11/2009
sampled: leg, eggs, mucus from skin and bucket
Also took weight: 94 g
2/4/2009
Sampled:
Removed portion of leg w/ razor blade
Removed egg strand (attached to wall facing camera): in -80
Mucus from skin
Mucus collected from bucket
Behavior: In corner with eggs in strange position. She is upside down, mouth and underside of tentacles facing up out of the water, with legs down around head and eggs (will take picture). Very difficult to capture with net and get into bucket. Usually she will try and swim away, but held on to side of tank and pushed net away. Wouldn't leave corner with eggs, took much longer than usual to capture. Once in bucket, she flipped upside down and didn't move. Didn't try to escape from bucket.
Once put back in tank, crawled down to substrate. Sat on bottom camouflaged to rocks. I dropped a mussel in, she grabbed it and pulled it under web.
Put green paper on end of tank (for better picture w/ web cam), tank probably a little darker now.
2/2/2009
Fed crab at around 11 am. Waited approximately 1 minute, then crawled down from corner with eggs and grabbed crab. Once caught, it brought the crab back up to the corner of the tank with the egg strands. Has been eating crabs for the last week, but not in front of me. Also, eats body of crab but leaves legs in tact. There are more eggs today, couldn't get an exact count but there are at least 15 strands now (only 7 or 8 before). She has been in the corner with her body covering the egg strands for most of the weekend, which could be explained by the fact that she laid more eggs.
To do: Submit abstract for undergraduate research symposium by Feb. 20
Found HSP 70 in tentacle, attempt to concentrate mucus and run western with HSP 70
1/30/2009
Articles about octopus reproduction/eggs: Itami et al. 1963, Marliave 1981, Snyder 1987, Boletzky 1989, Forsythe and Tol 1991, Villanueva 1995, Igleasias et al. 2004, Okumura 2005
1/28/2009
Prep of octopus leg sample for western blot with HSP 70. Sample: Collected on 10/24, portion of leg in tube with cell lytic, centrifuged, lots of bands showed up with coomassie stain. Combined 25 ul of liquid surrounding leg with 25 ul 2x SDS. In screw top tube labeled: octo leg, 1/28, 50 ul (blue tie attached to tube)
Sampled octopus. Removed from tank. Sucked mucus off skin with transfer pipet, removed mucus/water from bottom of bucket. When caught, octopus in corner of tank covering egg strands. Relatively easy to capture, held net next to her and inched closer until she was trapped inside. In bucket, same behavior as usual: Stayed in bottom, eventually tried to climb out. I got quite a bit of mucus from suction. Also collected one of the egg strands that was glued to side of tank (laid on 1/26). Put in tube, labeled, in -80. Also scraped green cement off side of tank, put in separate tube.
Now a total of 8 strands of eggs. Prior to today, 5 strands attached to tank. Yesterday sometime she released the other 3 strands that she was holding and glued to side of tank.
Octopus baby food: mysid shrimp, amphipods, crustacean appendages, crustacean larvae
1/26/2009
Octopus laid eggs (in AM or over weekend), looks like 5 strands total (2 stuck to side of tank, she is holding 3). Didn't sample.
1/21/2009
Sampled octopus:
Temp: 50-51 d F
Lights on for approximately 5 minutes before sampling.
Removed from tank, put in dry bucket.
Used transfer pipet to suction mucus off skin, got very small amount (labeled skin 1/21)
Octopus put back in tank, removed liquid (water+mucus) from bottom of bucket (labeled bucket 1/21)
Fed it a crab. Dropped in front of octopus, reached leg out to grab it, but crab ran across tank. Octopus didn't attempt to attack. Stayed very flat against bottom of tank.
In bucket was a light brown/red color. When put back in tank, turned very deep red. After 2-3 minutes, white spots appeared (after crab introduced).
1/14/2009
Octopus sampling plan:
Starting week of 1/19- This week only, sample on Wed because of holiday
next 3 weeks: Sample on Mondays, suction directly from skin, then collect mucus from bottom of bucket
Run gel on Wednesdays, take picture
Raise temperature on 2/10 (after 4 weeks of sampling under normal conditions) Raise temp by how much??
Now: water is 50 degrees F (10 C), raise to 60 F (= 15.5 C)
Continue sampling on Mondays, gels on Wednesdays
Every day when feeding (in addition to sampling days):
Record temperature
Record crab capture time
Notes on color, behavior
Questions:
Westerns and Sequencing: how often? when?
1/6/2009
Octopus mucus gel 1 (12/23/08) sequencing results
(P02662) Alpha-S1-casein precursor
Antioxidant peptide has 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity.
Subcellular location: Secreted
Tissue Specificity: Mammary gland specific, secreted in milk.
(P02663) Alpha-S2-casein precursor: Contains Casocidin-1
Casocidin-1 inhibits the growth of E.coli and S.carnosus
Subcellular location: Secreted
Tissue Specificity: Mammary gland specific, secreted in milk.
(P02666) Beta-casein precursor
Casoparan acts as a macrophage activator, increasing the phagocytic activity of macrophages and peroxide release from macrophages. It also acts as a bradykinin-potentiating peptide.
Antioxidant peptide has antioxidant activity.
Casohypotensin acts as a bradykinin-potentiating peptide. Induces hypotensin in rats. Acts as a strong competitive inhibitor of endo-oligopeptidase A.
Subcellular Location: Secreted
Tissue Specificity: Mammary gland specific, secreted in milk.
(P02668) Kappa-casein precursor: Contains Casoxin-C,6,A and B, and Casoplatelin
Casoxins A,B and C have opioid antagonist activity. Casoxin C causes biphasic ileal contractions through the binding to the complement C3a receptors.
Casoplatelin inhibits platelet aggregation.
Secreted in milk.
(P81605) Dermcidin precursor Preproteolysin: Contains survival promoting peptide DCD-1
Displays antimicrobial activity therefore limiting skin infection by potential pathogens in the first few hours after bacterial colonization. Highly effective against E.coli, E.faecalis, S.aureus and C.albicans
Secreted by eccrine sweat glands into sweat.
12/4/2008-
Continued remainder of trypsin digestion protocol started yesterday. At the final stage, samples were speed vac'd for two hours and then resuspended in 5uL of 5% acetonitrile/0.1% TFA and 5uL of 50% acetonitrile/0.1% TFA and stored @ -80C.
12/3/008 -
Destained excised bands (from gel on 12/1/2008) according to Invitrogen protocol. Performed trypsin digestion of excised bands according to Goodlett lab protocol. Incubated samples O/N at RT in rocker. Will continue rest of protocol tomorrow.
12/1/08
Repeat of 1D protein gel from 11/24 for sequencing:
1. Ladder
2. Mucus from bucket 30 (11/21)
3. Mucus from bucket 30 (11/21)
4. Underwater skin 40
5. Skin 40 (11/21)
6. Skin 40 (10/24)
7. Mucus from bucket 30 (10/24)
8. Mucus from bucket 30 (10/24)
Skin= transfer pipette, suction on octopus skin
Bucket= water+mucus collected from bucket after octopus removed
Silver Stain
Bands removed for sequencing: Total of 8

2 bands sequenced: Which ones?
11/26/08 -

Lanes
1. Ladder
2. 11/21 100bp
3. 11/21 200bp
4. 11/21 300bp
5. 11/21 400bp
6. 11/21 100, 200bp
7. 11/21 100, 200, 300, 400bp
8. 11/24 RT 100bp
9. 11/24 RT 200bp
10.11/24 RT 300bp
11.11/24 RT 400bp
12.11/24 RT 100, 200bp
13.11/24 RT 100, 200, 300, 400bp
14.11/24 100bp
15.11/24 200bp
16.11/24 300bp
17.11/24 400bp
18.11/24 100, 200bp
19.11/24 100, 200, 300, 400bp
20.11/24 Neg. Control
Results: Gel was run too long and 100bp and 200bp bands, though present, are not visible. :(
The first set of PCR (from 11/21) shows good amplification with distinct bands at each molecular weight. Unfortunately, we can't see whether or not distinct bands were produced in the mulit-product rxns (lanes 6 and 7) for all products (100 and 200bp bands). The second set of PCRs (from 11/24) show two different profiles. The first set that was run through the Opticon protocol shows a great deal of background (smearing, accumulation of non-specific bands), while the set (which came from the same master mix) that was run through the traditional thermal cycler shows nice, distinct bands.
11/24/08
Real time PCR, SeeGene Forever Ladder, made 50 micro reactions, split in two:
25 microliters ea. of 100, 200, 300, 400, 100+200, 100+200+300+400 bp template DNA for real time PCR
25 microliters ea. for regular PCR
1D Protein Gel:
New Samples collected on 11/21 and old mucus samples from 10/24 that didn't show up well w/ Coomassie stain
Bucket= mucus and water collected from bucket after octopus put back in water
Suction= transfer pipette on skin
Lanes:
1. ladder
2. 11/21 Bucket- 30
3. 11/21 Bucket- 30
4. 11/21 Underwater skin w/suction- 30
5. 11/21 Skin w/suction- 30
6. 10/24 Mucus from suction- 30
7. 10/24 Mucus from bucket- 30
11/21/08
Real time PCR, SeeGene Forever Ladder
Master mix: 8 rxns (50 micro ea.)
1 Primer 1
1 Primer 2
25 Immomix
1 Syto 13
Rxns:
2 microliters ea. of 100,200,300,400 templates
4 microliters 100+200, 8 microliters 100+200+300+400
Filled to 50 total microliters/tube w/ distilled water
2 controls w/ water no template
Melting curve not set up, not programmed to take any reads (no fluorescence measured, just PCR)
Sam set up melting curve after PCR
Sampled octopus: transfer pipettes
Tried to sample mucus w/ octopus still underwater, probably mostly sea water (2 tubes labeled "UW")
Put octopus in bucket, sucked mucus from skin (1 tube labeled "skin")
Octopus back in tank, sucked liquid from bottom of bucket, looked thick (2 tubes labeled "bucket")
11/14/08
Chelax in screw top tubes, just enough to cover bac loop (500)
5 tubes, one bac loop per tube
Incubate 30 min. at 96 degrees (vortexed after 20 min)
Spin in cold centrifuge for 10 minutes
Remove liquid from top
5 tubes labeled C-H (C is control)
PCR:
Primers- IGS 1392 F
235 125 R
2x Immomix- 12.5
PF- .1
PR- .1
Syto 13- 1
Template- 5
Water- 6.3
10/31/08
1D gel of samples collected on 10/24
10/24 sampling: Octopus removed from tank, placed in bucket. 5 sampling techniques:
1. Kimwipe rubbed on octopus, washed with cell lytic and spun: called "tissue"
2. Bac loop on octopus skin, multiple loops put into cell lytic: called "bac loop"
3. Mucus suctioned directly off skin w/ transfer pipette: called "mucus suction"
4. Mucus/water collected from bucket after octopus removed: called "mucus bucket"
5. Octopus arm segment: one was washed with cell lytic, one in water: called "leg w/wash and leg no wash"
Also will run: samples from gel on 10/20 concentrated w/ centricon (see method on 10/23), have retentate and filtrate from mucus and fecal sample
Gel
1. centricon mucus filtrate 40
2. centricon fecal retentate 40
3. centricon mucus retentate 40
4. leg no wash (B) 40
5. leg w/wash (B) 40
6. leg no wash (A) 40
7. leg w/wash (A) 40
8. mucus bucket (A) 40 Green tube
9. mucus suction 40
10. Bac loop 40
11. Tissue (A) 40 Orange tube
12. Ladder
Samples combined w/ 2x SDS, boiled 10 min, spun top speed for 2 min
SDS PAGE gel, 150 v, 45 min
| . 250 . 148 . 98 64 . 50 . 36 22 . 16 6 |
 |
| ................2................4.......5.....6......7..........................................12...... |
10/23/08 -
Mucus super samples A-E (from 10/17) were pooled and Fecal Super (from 10/13) and put through a YM-3 Centricon (3,000kDa) by spinning 7000RPM, 4C. There were two 2hr. spins (remaining pool samples were added to Centricon at each stop) and a final 8hr spin that went O/N.
10/20/08
"One of the advantages of Octopus as an experimental animal is that
successive muscle samples can be taken by cutting off the outer third of each arm in
turn, an apparently brutal treatment that has surprisingly little effect upon the
animals' behaviour. Our octopuses regularly fed within minutes of removing an arm
sample (without anaesthesia). A proportion of any wild-caught sample of octopuses
from the Mediterranean is found to have one or more regenerating arms, and the
animals are evidently well adapted to survive such mishaps."
"Reproduction versus somatic growth:
Hormonal control in octopus vulgaris"
R. K. O'DOR AND M. J. WELLS
Protein Gel, new mucus samples + feces (mucus collected on 10/17 w/ transfer pipette, feces on 10/13 right after octopus transferred to aquarium room)
4 mucus samples: A,B,C,D (All the same collection method, time, etc.)
- spin at top speed 20 min. (collect supernatant, keep pellet separate if present)
- 2x SDS
1 fecal sample ( will try several volumes)
- broke up clump of feces, spin 20 min
- run super
All: Boiled 10 min, cold centrifuge 2 min
Lanes
1. Ladder
2. Mucus super A 40
3. Mucus super B 40
4. Mucus super C 40
5. Mucus super D 40
6. Mucus super E 40
7. Fecal super 20
8. Fecal super 10
9. Fecal super (old) 30
10. Fecal precip (old) 20


 Bands from fecal super 10?
Bands from fecal super 10?10/17/08
Collected mucus samples from octopus (6 tubes total), used transfer pipette so mostly water.
Will spin and remove supernatant
10/10/08
"Senescence Marker Protein-30 Protects Mice Lungs from Oxidative Stress, Aging, and Smoking" http://171.66.122.149/cgi/content/abstract/174/5/530
10/3/08
Proposal Outline/Brainstorm (google docs)
9/29/08
"Optimization of sample preparation from skin mucus of a neotropical fish for two-dimensional substrate gel electrophoresis" http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6W9V-4KKNKYJ-3&_user=582538&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=582538&md5=b2f7995d5a0c4ca91e7c05590f405af4
9/24/08
"Mucins: structure, function, and role in pulmonary diseases" http://ajplung.physiology.org/cgi/reprint/263/4/L413.pdf
"Isolation and characterization of novel tachykinins from the posterior salivary gland of the common octopus Octopus vulgaris"
http://tinyurl.com/52zkhb
Pubmed seach "mucus protein skin" -
9/23/08
This AM pulled supernatant samples (2) from Centricon (very low volume). Place at 4C.
Two fecal samples still had liquid, centrifuged for another 3 hours @ 7100 rpms. -
Reverse spot spun two fecal samples. Notices precipitate (saved at 4C).
Put liquid in two new Centricons - spin for another 3 hours. prior to this they were subjected to 2+4+3=9 hours -
Spinning over: total 12 hours -
Bradford Assay:
Mucus
Harry 2: 18.57 ug/mL
New 2: 18.57 ug/mL
Feces
Super 1: 912.86 ug/mL
Super 2: 461.43 ug/mL
Protein Gel:
Mucus super (Harry 2, New 2): 25 uL + 25 uL 2x SDS
Fecal super (1 and 2): 75 uL + 75 uL 2x SDS
Fecal precip 1: 100 uL 1x SDS
Fecal precip 2: 50 uL 1x SDS
Lanes
1- Ladder 10
2- H2 30
3- H2 20
4- N2 30
5- N2 20
6- F Super1 30
7- F Super1 20
8- F Super2 30
9- F Super2 20
10- F Precip1 30
11- F Precip1 20
12- F Precip2 30
Coomassie stain for 1 hour
| . 250 . 148 . 98 . 64 . 50 . 36 . 22 6 . 4 |
 |
-
Picture flipped (fecal samples on left, mucus on right)
9/22/08
Remaining samples (Harry 2, New 2, 2 tubes of feces from New) centrifuged for 15 min. at high speed.
Liquid removed from top (into 4 tubes: H2 super, N2 super, New Feces super 1, New Feces super 2). Fecal samples divided, diluted with solution, centrifuged again for 10 min.
Put into Centricon tubes with water (mucus: 50% water, feces: 75% water) up to 2 mL, spin for 2 hours (initially)
At this point: 4 tubes of supernatant spinning in Centricon tubes, 6 tubes in freezer (2 mucus pellets, 4 fecal pellets)
9/17/08
Mucus was freeze-dried, approximately .04 grams.
Suspended half of sample (approx. .02 g) in 100 microliters 1X SDS.
Boiled 10 min, cold centrifuge 2 min.
Protein gel with various volumes.
Lanes
1: Ladder 8
2: 40
3: 30
4: 20
5: 5
6: 10
It looked like the dried sample was dissolved in the SDS, but when I centrifuged it something settled to bottom of tube (sample or something else?) I took the liquid from the top to load into wells, and centrifuged it again before I did the 5 and 10 microliter lanes. Some solid material most likely ended up in the gel.
Coomassie stain for 15 min.
| . . . . 98 kDa |
9/16/08
SDS Octopus Mucus Protein Gel
Protein gel w/ old octopus sample (GPO mucus SDS), we know it should produce several bands, intend to send bands for sequencing.
| . . . . . . . . . . A . . . B . C . . . D . . . . . |
 |
I cut 4 "bands" for sequencing, labeled from top of gel to bottom: A, B, C, D
A: 45 kDa (single band)
B: 60-80 kDa (smudge, multiple bands)
C: 80 kDa (single dark bank below smudge)
D: 148-250 kDa (smudged, multiple bands)
(very rough estimates, gel picture is upside down)
Re. Octopus rubescens care
"We have kept several species of octopus from O. cyanea to Hapalochlaena, but I think I now have a new favorite. Three months ago we collected a tiny O. rubescens with a mantle length under one cm. I put it in a 10 gal tank with a sand bottom and a small piece of pvc. The entire set-up was in a temperature controlled room kept at 15 C. The animal was very shy at first, but did eat large adult brine shrimp and tiny bits of raw shrimp. It gradually grew to about 2 cm in two months and was feeding on live ghost shrimp when I left for a month in the field. When I returned this weekend, the animal was 4 cm, ravenous, and runs down grass shrimp, crabs, stomatopods and just about any other live food we put in its tank. When I enter the room it displays and paces on the front wall of the aquarium. It was one of the easiest animals to rear we have ever had and looks to have a broad repertoire of displays. If you ever get a chance to rear one and can keep it in a cool tank, I would strongly recommend this species."
-from tonmo.com forum
07/28/08 Activity to Date Summary:
Three samples from two individuals were received from the aquarium. These included a fecal sample and mucus sample from a 6 kg female, and a mucus sample from a 2 year old male.
| external image File?id=d8jfhsm_4826frc4h8cr_b |
Figure 1
A 1:1 mixture of solid feces and SDS produced smeared bands after gel electrophoresis.
The mucus from the 6 kg female was concentrated using a Centricon tube with a YM-3 membrane. The resulting samples of molecular weight greater than 3000 and less than 3000 were labeled as samples #1 and #2, respectively. A 1:1 mixture of sample #1 or #2 and SDS produced no visible bands after gel electrophoresis. Sample #1 was concentrated using the centricon method for an additional 2 hours, resulting in the new sample #1.2. After a second protein gel containing both #1 and #1.2, and subsequent silver staining, a light band was visible at the same molecular weight for both samples (lanes 3 and 4 below). Three total protein gels for samples #1, #2, and #1.2 from the female octopus, resulted in no bands after coomassie staining, and one faint band after silver staining.
| external image File?id=ddvm3dkk_15cd5tnbfh_b |
Oyster tissue #1 #1.2 Pellet Mucus/SDS
Figure 2.
The mucus from the 2 year old male octopus was prepared three ways: a 1:1 mixture of liquid mucus and SDS, a 1:1 mixture of liquid mucus and LDS, and a 1:1 mixture of pelleted mucus and SDS. Each produced a faint, high molecular weight band. The SDS sample and pellet ran in two additional protein gels. The second gel, with coomassie staining, resulted in no bands for the SDS sample, and a light smear from the pellet. After the third protein gel and silver staining, both samples produced multiple indistinct bands (lanes 5 and 6 above).
To follow up on the successful silver staining of the SDS samples, a final protein gel was run with various volumes of sample. The mucus/SDS samples at 10, 15, and 20 microliters produced multiple, somewhat distinct bands. The mucus/LDS samples were also ran as a comparison, and it was determined that band clarity is greater with SDS. The samples #1 and #1.2 (centricon concentrated) again produced very faint bands. The supernatant from the pelleted sample (above picture) also produced multiple bands. The fecal samples were again smudged and indistinct. This final gel is a summary of my work with the first two octopus samples, the general goal being to detect protein bands in the mucus and clarify them.
|
||
| http://farm4.static.flickr.com/3243/2699095746_02ccebc506_o.png |
Figure 3.
