October 6, 2009
RNA Extraction
Background: Isolating RNA with TriReagent. RNA will be precipitated and washed in order to remove excess phenol and salt and then the RNA will be resuspended. Concentration of the sample (include multiplying by the dilution factor) will be calculated using absorption value. This will determine the purity of the RNA isolated.
Procedure
1. Add 500µL of the TriReagent to frozen sample tissue (50-100mg) in 1.5 mL tube.
2. Homogenize tissue with pestle
3. Add 500µL of the TriReagent to the tube again.
4. Vortex for 15 seconds and store at -80C
Observations
Isolating RNA from barnacle tissue that has experienced heat stress. Tissue sample was filled with shells and difficult to mix or homogenize efficiently. TriReagent was bright pink/red in color and remained the same color throughout entire experiment. Final tissue that was put into storage was still rather "chunky" and shell filled, however sufficient tissue was still broken up.
Protein Extraction
Background: Isolate cellular protein from whole tissue by using CelLytic MT. After extraction, the concentrations of proteins in sample will be determined. The Bradford Assay is a colorimetric assay that interacts with proteins; in a solution with proteins, the sample will turn different types of blue. The measured absorption value will determine the amount of protein present in the sample. A curve can be created using the formula from the Bradford Protein Assay Kit: y=1011.9x (R^2 = 0.9759).
Procedure
1. Add 0.5mL of CelLytic MT solution to frozen tissue sample (25mg) in 1.5mL tube.
2. Homogenize with pestle
3. Close and invert tube several times
4. Spin tube in refrigerated microfuge for 10 minutes at maximum speed.
5. Transfer supernatant to clean tube labeled with Protein, tissue type, initials, and the date
Quantification:
6. Dilute 15µL of protein sample and 15µL DI water, this will create a 1:2 dilution
7. Add 1.5mL of Bradford reagent
8. invert tube several times and incubate at room temperature for 10 minutes
9. Mix tube several times and transfer to 1mL plastic cuvette
10. Measure absorbance at 595nm and record the value
11. Measure again after mixing solution with pipette
12. Average the two absorbency values recorded
13. Store protein sample at -20C
14. Back calculate with Bradford protein assay kit curve
Observations
Barnacle tissue used, sample is from heat stressed barnacle. Most shell was removed, but some obstructed the homogenization. Sufficient amounts of tissue were still homogenized in tube. CelLytic MT solution was clear in appearance. Tube is labeled Protein, Barnacle HS, SH 10/6/09. 1:2 dilution was performed, and I will multiply total protein number by 2 in order to compensate for the dilution, after using the formula form the curve. Bradford reagent turned DI blank sample grey color and the protein sample color blueish. Error: I did not measure absorption twice, and therefore am unable to average the two values.
Results: Protein sample was absorption value was 0.386 A.
Use Bradford curve formula: y=1011.9x (R^2=0.9759)
x=absorption, y=Protein (ug/ml)
x=0.386 A
y=1011.9(0.386)
y = 390.5934 x 2 [to make up for dilution factor of 2]
y= 781.1868 ug/ml
The total protein value falls within the acceptable range, based on Bradford curve model. Next, I will determine which physiological characteristic of heat stressed barnacles to investigate further.
Lab 2: Tissue Extraction
October 13, 2009
RNA Isolation:
Need to isolate RNA in order to store an RNA stock.
Procedure:
1. Take sample from Lab 1 and incubate at room temperature for 5 minutes
2. Add 200 µl of chloroform to sample. Handle chloroform carefully under the hood
3. Vortex for 30 seconds, sample will become milky
4. Incubate at room temperature for 5 minutes.
5. Spin in refrigerated microfuge for 15 minutes
6. transfer clear liquid at the top into new tube - Labeled: RNA
7. Add 500 µl of isopropanol to the new tube with your RNA, invert until solution becomes uniform throughout
8. leave at room temperature for 10 minutes and then microfuge in refrigerator for 8 minutes at maximum speed
9. White pellet will form at the bottom, this contains the RNA and salts.
10. Remove supernatant and add 1mL of 75% EtOH to pellet and vortex briefly in order to dislodge the pellet
11. Spin in refrigerated microfuge at 7500g for 5 minutes
12. Remove supernatant, but be careful to not remove pellet; briefly spin to pool excess EtOH
13. Remove remaining EtOH and leave in open to let the rest evaporate
14. Resuspend RNA pellet in 100µl of 0.1% DEPC-H2O by pipetting up and down until pellet has dissolved.
15. Incubate for 5 minutes at 55C
16. Remove from heat and freeze, this is the RNA stock.
Results:
RNA was isolated from rest of tissue. This will serve as my stock RNA for further molecular investigation.
Conclusions/Next Steps:
The next step in the procedure is to do the RNA quantification.
Protein Gel:
Purpose is to separate proteins from one another based on the molecular weight. In the gel electorophoresis the mixture of proteins will be separated in an electric field.
Procedure:
1. Boil water on hot plate
2. Thaw protein extract from Lab 1 and invert several times.
3. In a new tube, add 15µl of protein sample and 15µl of 2X reducing sample buffer.
4. mix by flicking and briefly centrifuge (10 seconds), then boil the sample for 5 minutes
5. After boil, centrifuge for 1 minutes
6. Load sample into previously prepared well using the gel loading tip
7. Make sure to record which well contain which proteins and to include the marker: in this case the marker is SeeBlue Plus 2 Prestained standard 1X
8. Also record how much protein sample is put into each well based on the fact that there are 30µl of sample loaded into gel and the known µg/ml of protein sample
9. Put lid on gel box and plug in electrode, turn on power and run at 150V for about 30 minutes.
10. After gel has been run, remove lid and remove gel from gel box. Trim off wells and mark a corner to designate which way is up
11. Add 150mL of Coomassie stain and incubate for 5 minutes on rocker/shaker
12. Rinse with 10% acetic acid and incubate on shaker rocker for 15 minutes, change out the buffer and continue until gel run appears.
Results
Gel was a 4-20% Tris-HEPES SDS-PAGE and was run for 30 minutes at 150V. My sample of heat stressed barnacle proteins was put into well number 3. I put in 11.71µg of sample. Smaller samples of protein will run faster and will therefore show up farther from the well origin.
Conclusions:
Based on how much sample was put in and what proteins are expressed, we are able to see the sizes of proteins expressed from the sample based on the weight (kDa). Figure 1 is marker protein that has been loaded into well #1 in the image on the right. The right image Figure 2 is our group's protein. My heat stressed barnacle protein is in Well #3.
Darkest bands my well #3 are at approximately 250 and carbonic anhydrase kDa. Bands range over entirety of gel, 4-250dKa.
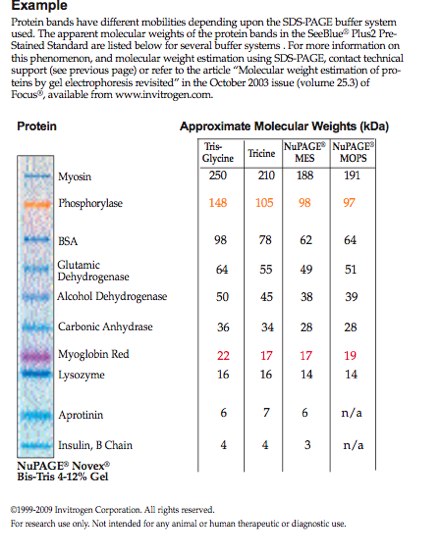 |
| Figure 1 |
Figure 1: Marker protein, SeeBlue 2 Ladder. NuPAGE MES gel molecular weights.
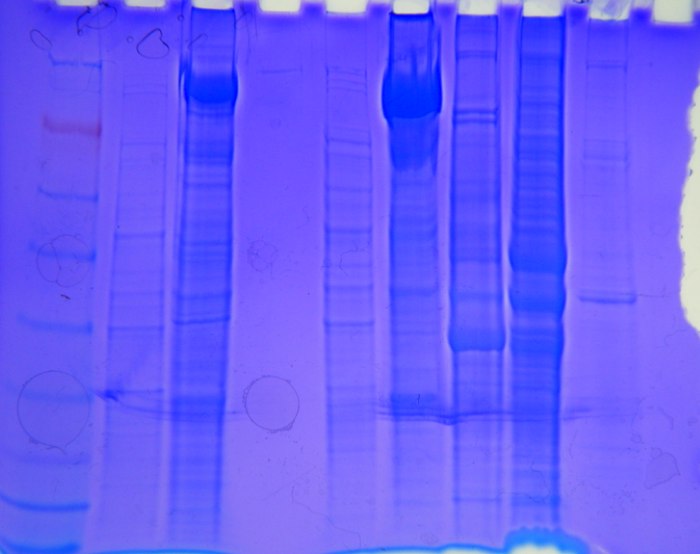 |
| Figure 2 |
Figure 2: Protein gel, marker is in well #1 on the far left (Figure 1). Volume put into each well was 30µl. From well #2 to well #9, left to right the samples were: oyster, barnacle heat stress, blank, unknown, heat stress barnacle, salmon brain, herring brain, and unknown.
Lab 3: Reverse Transcription and PCR
October 20, 2009
-
Purpose:
The purpose of this lab was to quantify the previously isolated RNA and reverse transcribe the RNA to produce complementary DNA. From this DNA a PCR amplification was performed.
RNA Quantification
Methods:
1. Keep RNA on ice at all times
2. Pipette 2µl of 0.1%DEPC-H2O onto the nanodrop and lower the arm - measure this as the "blank"
3. 2µl of RNA was put onto nanodrop and measured: record the A260 abs, RNA concentration, A260/280 and A260/320 ratios. The nanodrop uses the Beer-Lambert Law to calculate the RNA concentration
Observations:
From nanodrop, my RNA concentration was 91.8ng/µl,
260/280: 1.86
260/230: 1.12
A260: 2.294
A280: 1.232
Reverse Transcription
Methods:
1. Mix RNA stock by inverting
2. Transfer 25µg of RNA to new tube and add water so the total volume in 5µl.
3. Incubate tube at 75C for 5 minutes in thermal cycler
4. Transfer to ice for another 5 minutes
5. Make the master Mix, tube labeled: MM
Master mix ingredients, per reaction:
4µl 5x buffer (AMV RT Buffer)
8µl dNTPs (10mL total)
1µl AMV RTranscriptase
1µl Oligo dT Primer
1µl RNase free water
6. Add master mix to tubes with RNA and vortex
7. Incubate at RT for 10 minutes
8. Incubate at 37C for 1 hour in thermocycler
9. heat inactivate at 95C for 3 minutes
10. Spot spin and store at -20C
Observations:
This process yielded cDNA. 5µl of the RNA were added (step 2)
PCR:
1. Prepare and label 4 tubes; 2 for sample cDNA (S1 and S2) and 2 for negative control (B1 and B2)
2. Make the Master mix, make enough for pipetting error and following the concentrations below:
Ingredient Concentration: added volumes in ()
GoTaqGreen Master Mix, 1X (25µl)
Forward primer 0.1-1.0µM (2.5µl)
Reverse primer 0.1-1.0µM (2.5µl)
3. Total in each tube (4 total) will be 50µl, add 48µl of Master Mix. Add 2µl of sample to the S1 and S2 tubes and 2µl of water for the negative control tubes (B1 and B2)
4. Load samples into thermocycler
95C for 10 min
40 cycles
-95C for 30 sec
-55C for 30 sec
-72C for 90 sec
72C for 3 min
4C forever
-
Observations and results
Primers used for this PCR were primers to isolate specific heat shock proteins for heat stressed barnacle - Balanus
Label of primers: SH_Balanus_HSP_F and R
Forward primer: MW: 6,208.1; 29.8nm; GAGCGGCTGATCGGAGACGC
Reverse primer: MW: 6,079; 36.6nm: GATCTCCTCCGGCGCGAACG
Use formula: (C1)(V1)=(C2)(V2) to determine how much primer to add to master mix:
First add 100µL to primer tubes, nm value multiplied by 10 fold will give µM value of stock solution primer.
Use formula: (100µM)(x)=(10µM)(100µL) to determine how much stock primer should be added to make 100µL solution with 10µM concentration. x=10µL; add 90µL of DI water in order to obtain 100µL of 10mM solution.
Conclusions/Next Steps
RNA sample was clean, (A260/280=1.86). Reverse transcription yielded cDNA that was amplified via PCR with primers. The primers will isolate the Balanus amphitrite 70kDa heat shock protein. Next week we will run the PCR gel and hopefully see area isolated by primers.
Lab 4
10/27/09
Summary
The PCR product from Lab 3 will be put on a gel and analyzed. Protein samples that were run on gel from before will be transfered to membrane and probed with antibodies in order to identify the proteins.
Procedure - PCR on gel
1. Obtain gels made from last lab and put in gel box and will with 1X TAE buffer; remove combs carefully
2. Load 100bp ladder (7µl) in reference well (#1)
3. Load 25µl of each of the 4 tubes from previous PCR
Tube 1-4: S1= sample 1, S2 = sample 2, B1=control 1, B2 = control 2
My samples were loaded into Wells #2-5 on the bottom row of the gel
4. run gel at 100V for 1 hour, and then visualize
Results
Smearing of sample runs and a band appeared in control samples. This may be due to primer dimer issue, because bands are similar for both. Based on primers used, bp product size for Balanus HSP should be 216 bps long. See Figure 1 below.
Procedures - Transfer proteins to membrane: Western Blot
1. Cool transfer buffer to 4C, and soak filter paper, membrane, and gel in the Transfer Buffer for 15 minutes
2. Assemble blotting sandwich:
- Anode
- Filter paper
- Nitrocellulose membrane
-Gel
- Filter paper
-Cathode
3. Transfer blot for 30 minutes at 20 V
4. Remove the gel from the sandwich and rise with the transfer buffer and make sure to swab away any gel from the membrane
5. Stain membrane if necessary to see left over protein
Western Blot Protocol: use kit protocol
6. Prepare 20 mL of Blocking Solution with: Ultra filter water 14mL, Blocker Part A 4mL, and Blocker Part B 2mL: total volume will be 20 mL.
7. Put membrane in 10 mL of Blocking solution and cover in dish provided by kit
8. Decant blocking solution
9. Rinse membrane with 20 mL of water for 5 minutes and Decant, repeat again
10. Incubate membrane with 10 mL of Primary Antibody Solution overnight: Blocking Solution 10mL, HSP 70 antibody 3.3µl = total volume 10mL
11. Following day: Decant Primary Ab and keep at 4C.
See Figure 2 for Western Blot and FIgure 3 for gel.
Results:
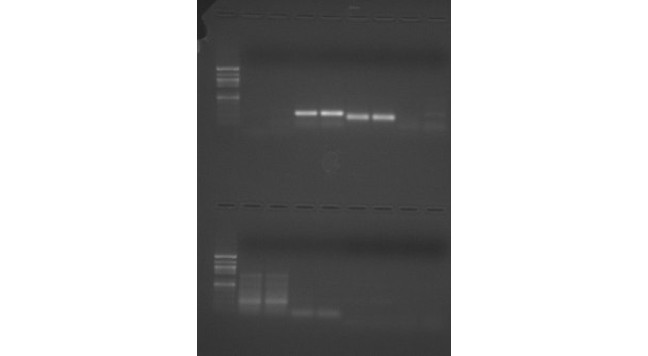
Figure 1: Gel from PCR. Top section: Well#1 is the ladder, 2&3 are Balanus negative control and 4&5 are balanus HSP samples. 6&7 are heat stresses balanus samples and 8&9 are negative controls. Bottom section: well#1 is ladder and 2&3 are heat stressed balanus samples and 4&5 are negative control samples. More intense bands in well 2&3 in the bottom section are approximately just over 200bps. The negative control wells 4&5 also have bands at approximately 200bps. My projected bps, based on chosen primers, should be 216 bps long. The bands that show up smeared in the sample wells and the bands that show up in the negative control wells are possibly due to dimer primer issue.
Figure 2: Western Blot image. Antibodies will show up with heat shock protein detection (HSP70). Well #1 is the ladder. Well 2 and 3 are heat stressed balanus and well 4 is balanus species. This figure shows that both heat stressed balanus tissue samples and not heat stressed balanus species code for HSP 70 protein.
Figure 3: Gel sample in which the protein samples were transferred to membrane.
Conclusions/Next Steps
The gel run from cDNA (Fig 1) sample yielded bands on all samples (S1 and S2 - Well#2 and 3 on the second row) including the negative controls (B1 and B2, Wells 4 and 5. The single intense band in the sample wells indicates that there is heat shock protein present in the heat stressed balanus tissue. My bp approximate length should be around 216 bps; bands show up somewhere between 200 and 300 bps. Smearing on either side of the sample bands and the fact that a band also showed up in the negative controls indicates possible error, extra PCR product formation, nonspecific primers, or primer dimer.
The Western blot image (FIg 2) shows that the antibodies bonded to HSP 70, and therefore the HSP 70 is present. Discrepancies in the intensity of all balanus species (heat stressed or not) HSP 70 bands may be due to differences in concentration of protein added. Figure 3 showed the protein on a gel that was used for the Western blot.
Next, a qPCR will be applied to the cDNA in order to determine the mRNA concentration.
Lab 5
11/3/09
Purpose:
The purpose of this lab was to perform a qPCR of the ctDNA-
Methods
1. Prepare master mix, make enough for 8 reactions.
Mix for 8 reactions:
2X Immomix 25µl, added 200µl to Master Mix, final concentration: 1x
Syto-13 dye (50µM) 3.5µl, added 28µl, final concentration: 2-5µM
upstream primer (10µM) 1.5µl, added 12 µl, final concentration: 0.1-1µM
downstream primer (10µM) 1.5µl, added 12 µl, final concentration: 0.1-1µM
Ultra pure water 16.5, added 132µl
2. Add 48µl of Master Mix to each tube in strip tube.
Labeled: 1:cDNA, 2: cDNA, 3: negative control, 4: negative control, 5: RNA to determine genomic DNA, 6: RNA to determine genomic DNA
3. Add 2µl of cDNA, negative control (Water), and RNA (diluted) to each strip tube.
Dilution for RNA is 1:4, added 15µl of water and 5µl of RNA for 1:4 dilution.
4. Cap wells securely, spin to let volume collect at the bottom
5. Clean lids before placing in Opticon
6. Load the plate and run the qPCR
Results:
My samples were in last well on the right (#12), from the top, two cDNA samples (1&2), two negative control samples (3&4), and two RNA samples (5&6) to test for genomic DNA carry over (not contamination..).
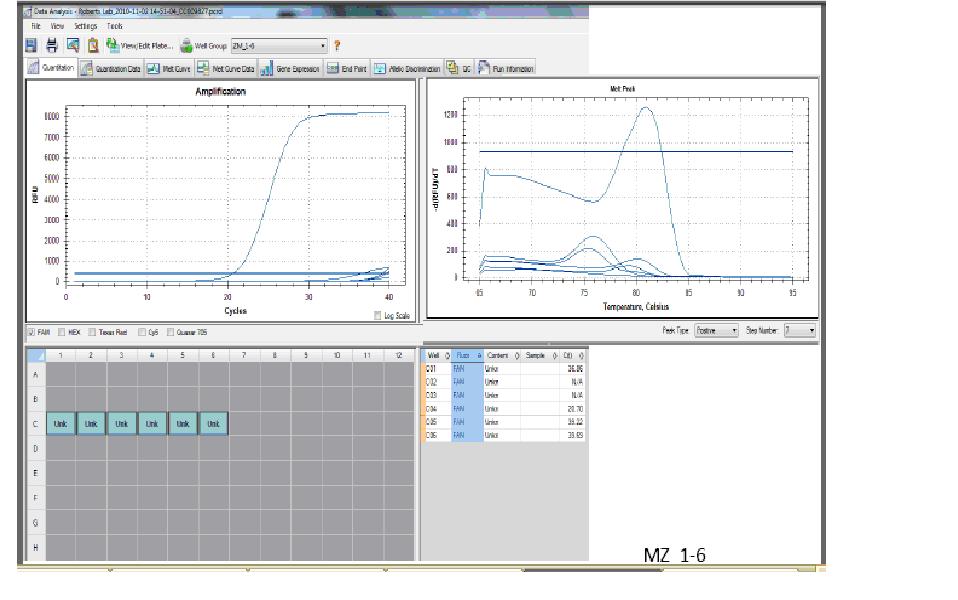
Figure 1:
qPCR results for cDNA (top 2 wells), negative control (next 2 wells), and RNA (bottom 2 wells) for HSP70 from heat stressed balanus.
Conclusions/Next steps
Figure 1 shows the qPCR results run. Based on the top right graph, peaks about the -1 Log of Flourescence show amplification of the sample. The top two wells, light blue and pink are cDNA amplification and the bottom two wells, green and red are RNA samples. These four samples show amplification, however the RNA sample with amplification indicates genomic DNA carry over. The negative control has no peak, yellow and dark blue, and indicates no contamination.
The bottom part of Figure 1 shows the peaks at which the raised temperature denatured the amplified samples. The initial peak most likely shows denaturation of the cDNA and the second peak, of lesser height, probably illustrates denaturation of the genomic DNA carry over. The second peak is at a higher temperature because the genomic DNA carry over would be longer due to entrons and higher amounts of hydrogen bonds to break.
Project report
11/10:
Supplies needed for week 1: 15 nudibranchs (hopefully) and 1 sea star (predator)sea water for 3 tubs, 1 for control, and 2 for treatments
1 M KCl, syringe/pipet, salinity meter, thermometer, scalpels, 1.5 mL snap tubes
- need to ID species, in order to get primers, ASAP
Primer:
RNA Extraction
11/17:-  macgavery Nov 17, 2009make sure to include DNAse and check for carryover prior to RT.
macgavery Nov 17, 2009make sure to include DNAse and check for carryover prior to RT.
RNA QUANTIFICATION
NOTE: Always keep your RNA samples on ice!
1. Pipette 2µL of 0.1%DEPC-H20 onto the Nanodrop pedistal and lower the arm.
2. Click "Blank", to zero the instrument
NOTE: steps 1 and 2 only need to be done once for the whole class.
3. Pipette 2µL of your RNA sample onto the Nanodrop pedestal and lower the arm
4. Click "Measure". Record your A260 absorbance, RNA concentration (ng/µL), A260/280 ratio and A260/320 ratio.
NOTE: The Nanodrop uses the Beer-Lambert Law to calculate RNA concentration for you. See Lab 1 notes on RNA extraction for more information on the calculation and how to evaluate RNA purity using A260/280 and A260/A320 ratios.
5. Raise the arm and wipe off you sample with a Kim Wiple
6. Clearly label your stock RNA sample with the word "RNA", source organism/tissue, your initials, today's date and the concentration in ug/uL.
7. storage at -80C.
REVERSE TRANSCRIPTION PROTOCOL
1. Mix your stock RNA sample by inverting tube several times.
2. Transfer 25ug of your RNA (.25ug of mRNA) to a fresh PCR tube. Bring the volume up to 5uL with PCR water. If necessary, spin tube briefly to pool liquid.
3. Incubate tube at 75C for 5mins in thermal cycler.
4. Transfer tube IMMEDIATELY to ice and incubate for at least 5mins.
5. Make Master Mix (MM)
PER RXN
4 ul 5x Buffer (AMV RT Buffer)
8 ul dNTPs (10 mM total)
1 ul AMV RTranscriptase
1 ul Oligo dT Primer
1 ul RNase free water
Total = 15 ul
- Add MM to tube with diluted RNA in it (total volume now 20 ul)
- Vortex
- Spot spin
- Incubate at RT for 10 min
- Incubate at 37C for 1 hr in thermocycler
- Heat inactivate @ 95C for 3 min
- Spot spin
- Leave cDNA on ice or store at –20C
PCR-
Polymerase Chain Reaction involves amplifying a DNA (genomic or complementary) target using a polymerase, primers (short oligonucleotide), and dNTPs (A, C, T, Gs). In general the reaction is placed in a machine (thermocycler) where a series of temperature changes are performed [Denature (~94C), Anneal (primer specific ~50-60C), and Extention (~72C)].
For this lab you will be using Promega's GoTaq Product. Please Read!
Prepare your samples in duplicate AND make sure to include at least 2 negative controls for each primer (no template).
For a 50μl reaction volume:
| Component |
Volume |
Final Conc. |
| GoTaq®Green Master Mix, 2X |
25 |
1x |
| upstream primer, 10μM |
0.5–5.0μl |
0.1–1.0μM |
| downstream primer, 10μM |
0.5–5.0μl |
0.1–1.0μM |
| DNA template |
1–5μl |
<250ng |
Load reactions into thermocycler.
- prepare Agarose gels here. (Plan on making them earlier- maybe outside of lab time, so we can run PCR here. If not, run next week (11/24)
11/24:
Run PCR products on gel1. place gel in gel box and fill with 1x TAE buffer (to fully cover wells)
2. remove combs from wells
3. load 7uL 100bp ladder in far left lane
4. load 25uL of your PCR sample into the gel (retain the remaining vol at -20ºC)
5. run gel at ~ 100V for ~ 1hr
6. visualize the gel on the UV transilluminator
Analyze results
11/10/09 Lab, Day 1 of project Nudibranch defense
For KCl solution:
74.553 g/mol if molecular mass of KCl
1.864g of KCl in 25 mL of DI water
Start with KCl shock. Measuring 1 liter of sea water in open glass tank.
Acclimate for a bit, and add 1M KCl.
Seawater from same source, and will be using same seawater for each treatment.
Original housing salinity is 38ppm
Table 1. Labeled tubes. Also record where the samples are stored (temperature and location in lab)
||
| Types of treatment |
Control |
KCl |
Predator |
|
| Labels: |
1,2,3,4 |
A, B, C |
D, E, F |
|
| RNA extraction |
RNA 1, 2, 3, 4 Stored at -80C |
RNA A, B, C Stored at -80C |
RNA D, E, F Stored at -80C |
|
| RNA isolation |
||||
| RNA quantification |
||||
| Table for Observations: Table 2: KCl observations, A-C Observations for before sample was taken and approximate time (sec) after KCl shock was induced. All samples are in 1L seawater to begin Nudibranchs have been sitting in tanks for an least ten minutes to acclimate 1 M KCl that was mixed has salinity at 55ppm Taking about 50 mg of tissue sample from head. Tissue samples are suspended immediatley in trireagent, 1mL. Matched videos and images. |
||||
| A |
B |
C |
||
| Start Salinity: 32ppm 2:28pm Video 1 Initial salinity: 35ppm Observations: see Video 1, specimen A rolled up away from place of origin and is still freaking out... Picture 1 (after videos) 153 0237 Sample taken at 16:37 |
Start Salinity: 32ppm 2:30pm Video 2 Initial salinity: 34ppm Same observation, quickly rolled up away from origin of KCl Picture 2 sample taken at 17:33 |
Start Salinity: 32ppm 2:32 Video 3 initial salinity:34ppm same observation, see video 3 Picture 3(after videos. Sample taken at 17:27 |
||

Issues:
-possibly stress right before dissection...?
-carry over of seastar residue or nudibranch residue
-sea star my not be hungry for our nudibranchs...
hopefully nudibranch will be scared
Table 3: Predator response observations for D-F. Time taken after sample taken (Sec). Also record which video number correlates which each sample.
| D |
E |
F |
||||
| Start salinity:32ppm time:2 min 38 Video #4 observations at how many seconds in: Nudibranch and seastar make initial contact and nudibranch seems to go away, and then seastar makes an advance... nudibranch may be stinging the seastar. |
Start salinity:32ppm time:7:38 Video #5 & 6 Similar observations from D. |
Start salinity:32ppm time:2:54 Video #7& 8 (mostly 8) Similar observations from D and E. |
||||
| RNA extraction observations: - difficult to homogenize, gooey substances. - possible that homogenizing was improper, and removal of supernatant was not removed cleanly - possible steps of contamination. |
||||||
|---|---|---|---|---|---|---|
| RNA extraction procedure: 1. Add 500uL of TriReagent to a 1.5mL snap cap tube. Store on ice. 2. Using a clean razor blade, cut a piece of frozen tissue weighing between 50-100mg and add to tube containing TriReagent.* *to save time, this step has been performed for you. 3. Carefully homogenize the tissue using a disposable pestle. 4. Add an additional 500uL of TriReagent to the tube and close the tube. 5. Vortex vigorously for 15s. 6. If we run out of time we can store at -80C 7. Incubate tube at room temperature (RT) for 5 mins. 8. In the fume hood, add 200uL of chloroform to your sample and close the tube. NOTE: Due to the high volatility of chloroform, pipetting needs to be done carefully and quickly. Have your tube open and close to the container of chloroform before drawing and chloroform into your pipette tip. 9. Vortex vigorously for 30s. You are vortexing correctly if the solution becomes a milky emulsion. 10. Incubate tube at RT for 5 mins. 11. Spin tube in refrigerated microfuge for 15 mins. @ max speed. 12. Gently remove tube from microfuge. Be sure not to disturb the tube. 13. Slowly and carefully transfer most of the aqueous phase (the top, clear portion) to a fresh microfuge tube. Do NOT transfer ANY of the interphase (the white, cell debris between the aqueous and organic phase). 14. Close the tube containing the organic and interphase and properly dispose of the liquid inside the tube as well as the tube itself at the end of the lab. 15. Add 500uL isopropanol to the new tube containing your RNA and close the tube. 16. Mix by inverting the tube numerous times until the solution appears uniform. Pay particular attention to the appearance of the solution along the edge of the tube. If mixed properly, it should no longer appear viscous/"lumpy". 17. Incubate at RT for 10 mins. 18. Spin in refrigerated microfuge at max speed for 8 mins. 19. A small, white pellet (RNA and salts) should be present. If not, do not fret. Continue with procedure. 20. Remove supernatant. 21. Add 1mL of 75% EtOH to pellet. Close tube and vortex briefly to dislodge pellet from the side of the tube. If the pellet does not become dislodged, that is OK. 22. Spin in refrigerated microfuge at 7500g for 5mins. 23. Carefully remove supernatant. Pellet may be very loose. Make sure not to remove pellet! 24. Briefly spin tube (~15s) to pool residual EtOH. 25. Using a small bore pipette tip (P20 or P200 tips), remove remaining EtOH. 26. Leave tube open and allow pellet to dry at RT for no more than 5mins. 27. Resuspend pellet in 100uL of 0.1%DEPC-H2O by pipetting up and down until pellet is dissolved. 28. Incubated tube at 55C for 5mins. to help solubilize RNA. 29. Remove tube from heat, flick a few times to mix and place sample on ice. This will be your stock RNA sample. 30. Quantitate RNA yield using spectrophotometer (we will be using the Nanodrop). |
||||||
Store at: -80C
Observations
Step up for treatments:

Nudibranch:

Salinity shock physical response

Sea star and nudibranch

Next steps
Will be picking primers as soon as possible.
Next week we will be doing RNA isolation and quantification and hopefully preparing agarose gel for later PCR gel run. (-
11/17/09, Day 2 of project nudibranch defense.
- today, from tissues and trireagent, we will take our isolated RNA, and we will add another step of DNAase procedure before making our cDNA.
DNAase procedure
- enzyme that will break up any doubled stranded DNA, always keep on ice
0.5mL tubes
1) 2.5uL DNA ase buffer 1uL turbo DNAase and 20.5uL of RNA to make total of 24uL at 37C for 30 minutes
2)+1uL TUrbo DNAase and put at 37C for 30 minutes
3) 2.5uL inactivation reagent, RT for 2 minutes with mixing
4)spin at 10,000rcf for 1.5 minutes
5) transfer supernatent to a new tube and spec, normalize with 0.1% DEPC H2O
RNA QUANTIFICATION- perform dilutions so lowest spec-ed concentration is uniform with all of them....
(normalize with 0.1% DEPC H2O) - dilution: C1V1=C2V2... C2 will be lowest RNA concentration and figure out all the rest accordingly
Spec result is C1, V1 is unknown, C2 is lowest RNA concentration and V2 option (lower amount)
C1V1 = C2V2
(1127 ng/uL) (V1) = (227.06 ng/uL)(20 uL)
V1= 4 uL
New tube: total of 20 uL: 4 uL of RNA, 16 uL water
Table 4: Table summarizes concentrations and dilution factors. Each dilution total volume will add up to 20μl. Each volume below is in μl.
| Sample ID |
Concentration |
RNA for Dilution |
Water for dilution, total is 20 μl |
|||||
| 1 |
838.84 |
5.41 |
14.59 |
|||||
| 2 |
227.06 |
20 |
0 |
|||||
| 3 |
415.51 |
10.9 |
9.07 |
|||||
| A |
744.44 |
6.10 |
13.9 |
|||||
| B |
364.59 |
12.4 |
7.54 |
|||||
| C |
287.47 |
15.80 |
4.2 |
|||||
| D |
1127.51 |
4.03 |
15.97 |
|||||
| E |
387.27 |
11.73 |
8.27 |
|||||
| F |
599.79 |
7.57 |
||||||
| 12.43 |
NOTE: Always keep your RNA samples on ice!
1. Pipette 2µL of 0.1%DEPC-H20 onto the Nanodrop pedistal and lower the arm.
2. Click "Blank", to zero the instrument
NOTE: steps 1 and 2 only need to be done once for the whole class.
3. Pipette 2µL of your RNA sample onto the Nanodrop pedestal and lower the arm
4. Click "Measure". Record your A260 absorbance, RNA concentration (ng/µL), A260/280 ratio and A260/320 ratio.
NOTE: The Nanodrop uses the Beer-Lambert Law to calculate RNA concentration for you. See Lab 1 notes on RNA extraction for more information on the calculation and how to evaluate RNA purity using A260/280 and A260/A320 ratios.
5. Raise the arm and wipe off you sample with a Kim Wiple
6. Clearly label your stock RNA sample with the word "RNA", source organism/tissue, your initials, today's date and the concentration in ug/uL.
7. storage at -80C.
Next steps:
Will be coming into lab Thursday 8AM to perform dilutions and do reverse transcription. Goal is to run gel next week, therefore may be taking additional lab time for qPCR.
Use Primers:
SA_aeolidia_COI_F
GAGGGAGGGGCTGGGACAGG bp# 20 melt@ 59.96 A. papillosa Cytochrome C Oxidase Subunit I AY345028.1 261
SA_aeolidia_COI_R
TGCCCCTGCCAAAACAGGCA bp# 20 melt@ 59.39 A. papillosa Cytochrome C Oxidase Subunit I AY345028.1 261
SA_aeolidia_HSC70_F
GCCGGAGACACTCACTTGGGA bp# 21 melt@ 58.17 A. papillosa Heat Shock Cognate FJ753654.1 126
SA_aeolidia_HSC70_R
CCTCAGACGTCGCACAGCCC bp# 20 melt@ 59.77 A. papillosa Heat Shock Cognate FJ753654.1 126
SA_aeolidia_HSP70_F
ACTCCCTGGCGGAGAAGGAGG bp# 21 melt@ 59.36 A. papillosa HSP 70 FJ232695.1 128
SA_aeolidia_HSP70_R
ACCCTTGCTGGACCCACCCT bp# 20 melt@ 59.37 A. papillosa HSP 70 FJ232695.1 128
11/19/09 Day 3, Extra lab time 8AM Thursday morning
- perform dilutions, and perform reverse transcription.
Dilutions: see below for chart of dilutions
-make stock primer solutions, to be stored at -20C
RNA transcription
Methods:
1. Mix RNA stock by inverting
2. Transfer 25µg of RNA to new tube and add water so the total volume in 5µl.
3. Incubate tube at 75C for 5 minutes in thermal cycler
4. Transfer to ice for another 5 minutes
5. Make the master Mix, tube labeled: MM
Master mix ingredients, per reaction: (total put into Master Mix solution in parenthesis)
4µl 5x buffer (MMLV RT Buffer) (40µl)
8µl dNTPs (10mL total) (80µl)
1µl MMLV RTranscriptase (10µl)
1µl Oligo dT Primer (10µl)
1µl RNase free water (10µl)
Total for each is 15uL
Total volume after added MM will be 20 uL
6. Add master mix to tubes with RNA and vortex
7. Incubate at RT for 10 minutes
8. Incubate at 37C for 1 hour in thermocycler
9. heat inactivate at 95C for 3 minutes
10. Spot spin and store at -20C
Next Steps:
Perform the qPCR from the cDNA made last week.
11/24/09 Tuesday
qPCR performed at 4:00pm with the cDNA made from last week.
- possibly making the gel for electrophoresis next week.
Methods:
1. Master Mix was prepared with the following recipe for 10 reactions for a total of 48 uL per reaction, 2uL of sample of control...
10 reactions desired.. make master mix for 12 reactions.
a. 300uL Master Mix, 2X (Immomix): 25 uL
b. 42uL Syto-13 dye (50uM): 3.5 uL
c. 18uLUpstream primer (from previous stock): 1.5 uL
d. 18uL Downstream primer (from previous stock): 1.5 uL
e. 198uLUltra Pure Water: 16.5 uL
2. 48 uL of Master Mix were added to strip tubes
3. 2uL of cDNA for each primer was added to the wells
4. 2uL of pure water were added for the blank negative controls
5. The plate was loaded onto a real-time PCR thermocycler.
Well labels and locations are recorded. qPCR results will be looked at tomorrow morning
Results and next steps:
Need to perform a duplicate qPCR, timing unsure right now
Results from qPCR are inconclusive. Contamination is present probably in water sample or during some molecular procedure.
Curves are irregular and there is no pattern.
Tuesday 12/1/09
A second qPCR was performed
Same procedure from above:
COI primer samples were diluted 1:10 because that may be the source of the irregular curves
Results:
Results from the second qPCR are again, irregular. Contamination is present and amplified curves are not in any identifiable pattern.
Possible reasons:
- contamination
- wrong primers
- wrong species
- wrong tissue samples taken
- molecular technique incorrect from the begining
Next steps:
- Run a gel, this may tell us if anything is even there, possible that we have incorrect tissue.
Friday - 12/4/09
- Run a gel and interpret
Procedure:
1. weigh 2g of agarose and mix with 150mL 1x TAE in a 1L flask
2. microwave solution for ~ 3 minutes (less than 3 minutes- approx. 1:45 min)
3. cool solution (you should be able to touch the flask for a few seconds), then add 12uL ethidium bromide.
4. mix thoroughly by swirling, then pour into gel tray.
5. add gel combs
6. after gel is set, wrap in plastic wrap and place gel in the fridge, sit for at least 1 hour
7. Load samples:
On the far left well, added 20uL of 100bp ladder
Added 5uL loading die to each qPCR product that was 50uL each
From left to right:
Ladder, 1, 2, 3, A, B, C, D, E, F samples
Results:

Our samples were run in the top section of the gel.
There is no conclusive banding, there is only "intense" primer dimer
Next steps:
Time is a limiting factor.
Ideally, we would like to either duplicate the experiment and homogenize entire nudibranch to ensure that the correct RNA was extracted. GenBank did not have a sufficient number of available primers, possibly use a different species, or approach with a different method for measuring stress response.
Or, with our original RNA, we could perform dilutions again and make more cDNA and run a few more qPCRs and see if there was just a problem with contamination.